Res-Q Platform redesign
Designing for precision, compliance, and clarity in life sciences software validation
Client: Sware
Duration: Jan – May 2024
Usability TestingUI DesignWeb DesignDesign System
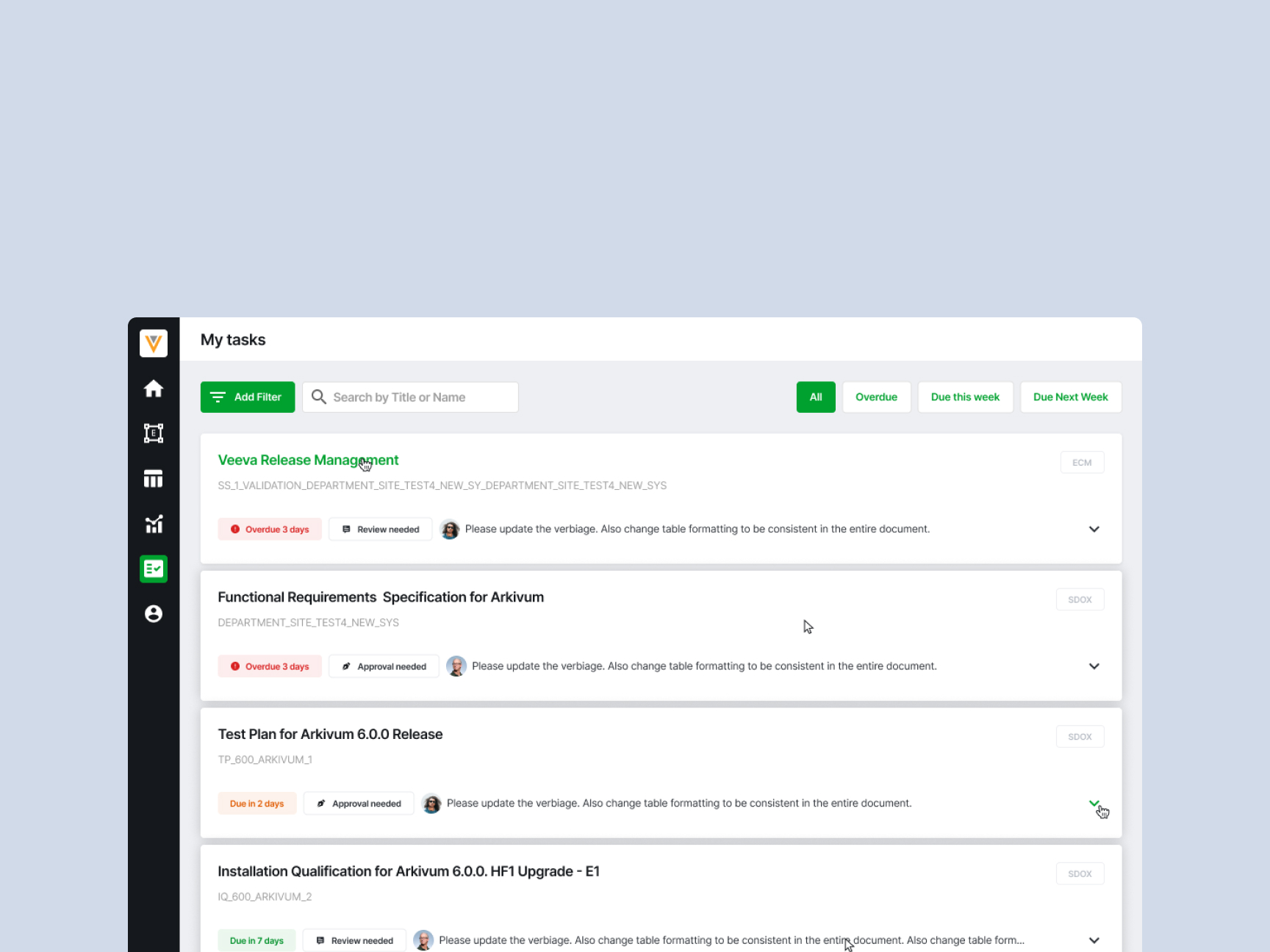
The problem
Compliance experts at Res_Q struggled to manage and track complex validation workflows and documents, often relying on external tools.
The solution
I led the redesign of critical features like Priorities of Change and the Tasks page, streamlining navigation, filtering, and task visibility while improving UI consistency.
Impact
Reduced confusion around task ownership. Helped pitch the feature to new clients. Improved adoption by internal validation teams.
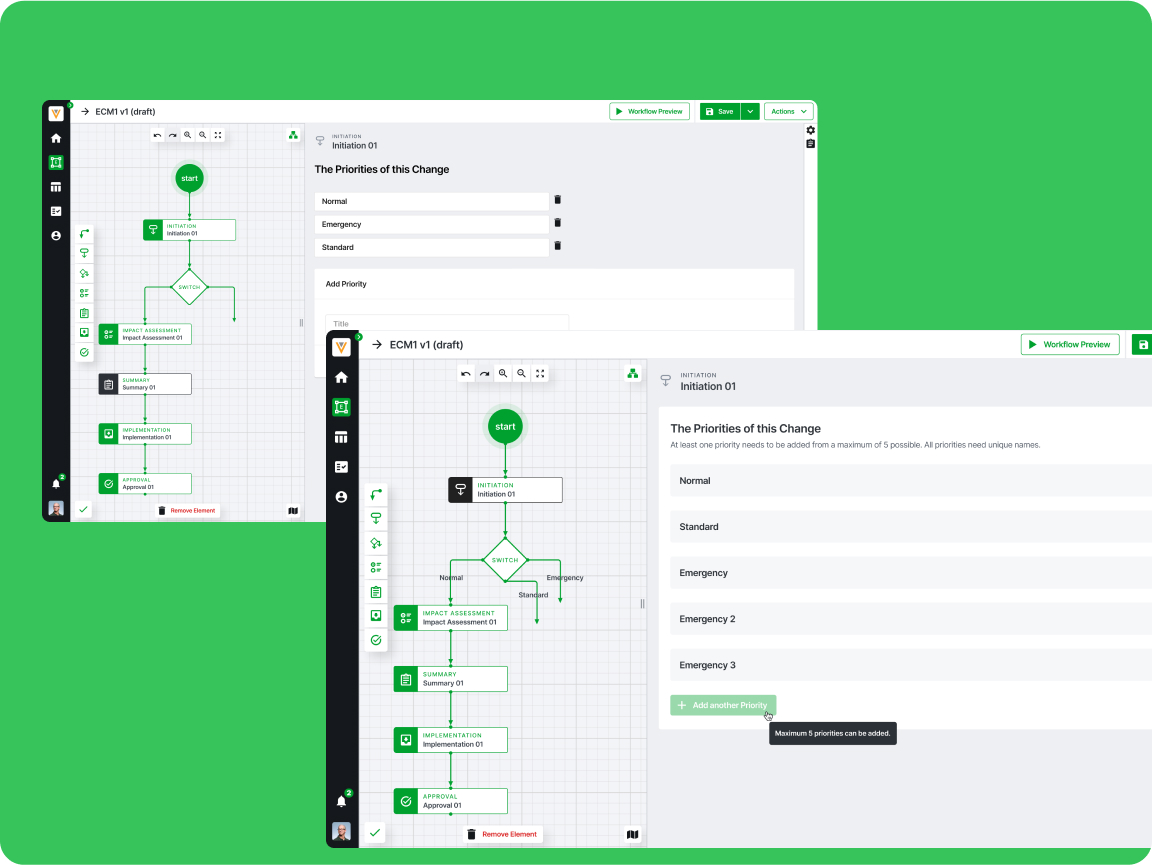
Efficient Workflows
Tools were optimized around team use-cases and multi-platform compatibility.
Challenges
Life sciences companies face extreme regulatory scrutiny. The Res_Q team had:
- Multiple document types per project (often 1000+)
- Complex workflows requiring absolute traceability
- UI inconsistencies due to previous resource constraints
- Internal stakeholders using external tools to compensate for missing UX functionality
Approach
Outcome
- Improved user satisfaction (measured via stakeholder interviews and internal feedback)
- Platform is now more sellable to enterprise clients due to increased UI consistency
- Exposed deeper platform issues: laid groundwork for future system design and modular UI
- Bonus win: Stakeholders adopted my usability insights to plan a design system revamp